If you or a loved one developed a meningioma brain tumor after using Depo-Provera, you may be entitled to significant financial compensation. The Depo-Provera lawsuit targets Pfizer Inc. for failing to warn users about the risk of brain tumors linked to long-term use of this injectable birth control. Clinton O. Middleton, Attorney at Law, is dedicated to helping women across the US seek justice.
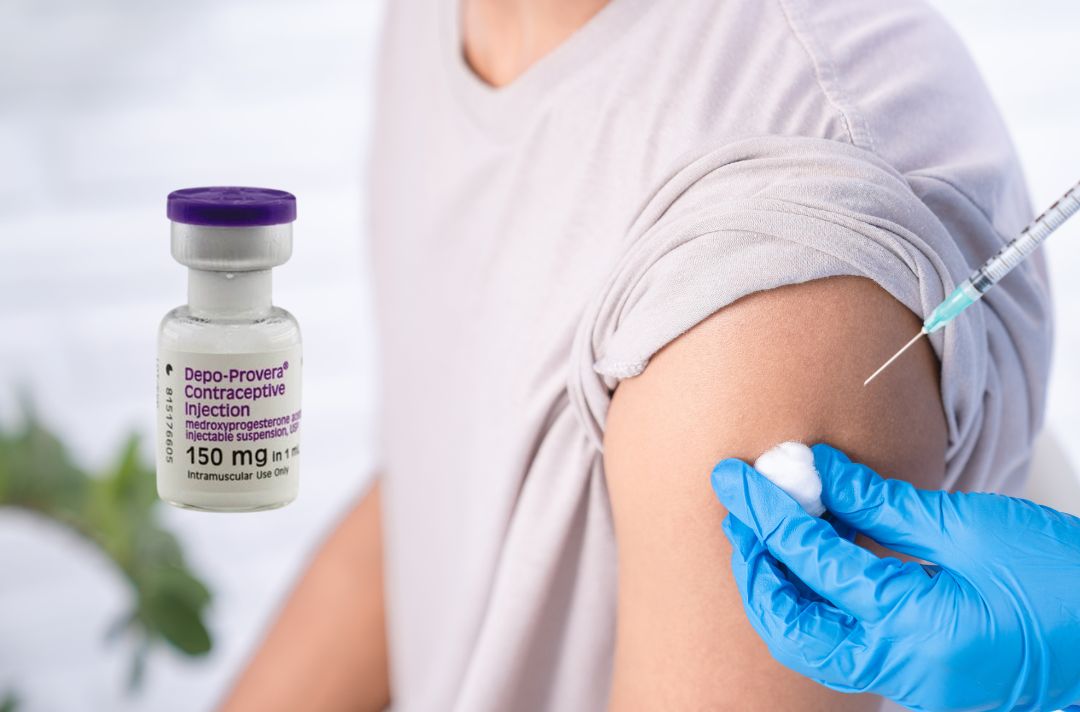
Depo-Provera, a popular contraceptive injection containing medroxyprogesterone acetate, has been linked to a 5.6-fold increased risk of meningioma brain tumors in women using it for over a year, according to a March 2024 study in The BMJ. Lawsuits allege that Pfizer knew or should have known about this risk but failed to update US warning labels, unlike in Canada and the EU, where meningioma risks have been recognized since 2015.
The MDL streamlines cases for efficiency, allowing plaintiffs to share evidence while maintaining individual claims. Victims are seeking compensation for medical bills, lost wages, and emotional distress caused by meningioma diagnoses.
Filing a Depo-Provera lawsuit can hold Pfizer accountable and provide financial relief for:
You may be eligible to file a lawsuit if you meet these criteria:
Most states impose a 2–3 year deadline from the date of your meningioma diagnosis to file a lawsuit, although some states allow only one year. An experienced Depo-Provera lawsuit attorney can assess your case and identify any exceptions to extend filing deadlines.
Navigating a Depo-Provera lawsuit requires legal guidance to counter Pfizer's robust defense teams. Clinton O. Middleton, Attorney at Law, offers personalized, compassionate representation with no upfront costs.
March 2024: A BMJ study links Depo-Provera to a 5.6 times higher risk of meningioma.
October 2024: First lawsuit filed in California.
February 2025: MDL was formed in the Northern District of Florida.
July 2025: MDL grows to 435 cases; Pfizer submits 8,000+ documents.
Depo-Provera's active ingredient, medroxyprogesterone acetate, mimics progesterone, which can stimulate meningioma growth in hormone-sensitive tissues. Meningiomas form in the meninges, the protective layers around the brain and spinal cord, and can cause severe symptoms:
Approximately 10–15% of meningiomas are cancerous, requiring aggressive treatments like surgery or radiation. The BMJ study found that women using Depo-Provera for over a year face a significantly higher risk compared to non-users.
When selecting Depo-Provera lawsuit lawyers, experience and dedication matter. Clinton O. Middleton offers:
What are the grounds for a Depo-Provera lawsuit?
Lawsuits claim Pfizer failed to warn users about the risk of meningioma brain tumors, despite evidence linking long-term use to a 5.6x higher risk.
How much can I expect from a settlement?
Settlements may range from $100,000 to $1M+, depending on tumor severity, medical costs, and lost wages. Past meningioma cases averaged $800,000.
How long do I have to file a claim?
Most states allow 2–3 years from diagnosis, but some have a 1-year limit. Contact an attorney immediately to avoid missing deadlines.
Can I file if I used a generic version of Depo-Provera?
Yes, lawsuits include authorized generics like those from Greenstone or Viatris, as they contain the same active ingredient.
What evidence do I need to file?
Medical records confirming Depo-Provera use and a meningioma diagnosis are critical. Our team can help retrieve records from pharmacies or providers.
Don't let Pfizer's negligence go unchallenged. If you or a loved one developed a meningioma after using Depo-Provera, Clinton O. Middleton, Attorney at Law, is ready to fight for your financial compensation. With a proven track record and a commitment to justice, we'll handle every aspect of your case while you focus on recovery. Call (703) 646-8024 or fill out our contact form for a free, no-obligation case review today.